
April 1 (UPI) — Meridian Medical Technologies, a manufacturer of Mylan’s EpiPen auto-injectors, has expanded a recent voluntary recall to include some of the life-saving medical devices in the United States, officials said Friday.
Mylan’s original March 20 recall affected about 80,000 of the EpiPen devices shipped to locations outside the United States.
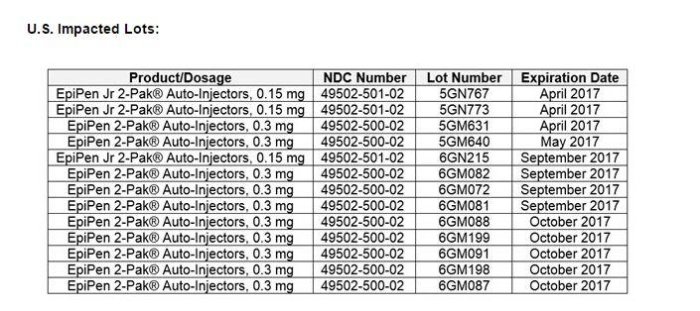
Mylan NV announced that the additional recalled items were part of 13 lots of EpiPen (0.3 mg) and EpiPen Jr. (0.15 mg) 2-Pak Auto-Injectors that carry expiration dates between April and October. Mylan brands adult versions with a yellow label and junior devices with a green label.
The company said the recalls are the result of two reported EpiPen failures that occurred outside the United States — due to a “potential defect in a supplier component” — and that more than 80,000 auto-injectors could contain the fault.
“The potential defect could make the device difficult to activate in an emergency and have significant health consequences for a patient experiencing a life-threatening allergic reaction,” Mylan said in a statement Friday.
EpiPens administer doses of epinephrine to patients with anaphylaxis — a serious allergic reaction that can be fatal if not treated immediately.
Mylan said both reported failures included devices, manufactured between December 2015 and July 2016, that were already included in the original recall. No device failures have been reported in the United States.
“The expanded voluntary recall is being initiated in the U.S. and also will extend to additional markets in Europe, Asia, North and South America,” Mylan said.
The recalled items will be replaced at no cost. The company said the action is being done with the knowledge of the U.S. Food and Drug Administration.
Customers should contact Mylan at (800) 796-9526 or email [email protected] if they have any questions.
“We are asking patients to keep their existing product until their replacement product can be secured,” the company said.







LOL! Absolutely brilliant move by Pfizer and Mylan! Protect your market share by recalling the sky high priced product close to the expiry date and replace it for free! Where do these people come up with these schemes? Now all the competitors who were ramping up for the busy season will be left with large amounts of stock that cannot be sold and will expire worthless! LOL! What a bare knuckle tactic. WOW!
Odds of failure per 80000 are .0000246