New Process May End Water Shortages
Did you know less than one percent of Earth’s water is drinkable?

With that being said, water is our most precious commodity on the planet. In fact third world countries are constantly suffering the ravages of either not enough water or polluted water.
Experts are saying within a few years we will have far less drinking water in reserve than we have now.
Natural droughts, usage and other factors take its toll on our water supply every year.
One only needs to look to California to see the impact a major drought can have on a population.
For years the idea of desalination, removing salt and other minerals from our biggest available source of water the ocean, may be the key to help satisfy the growing thirst for fresh water for drinking, farming, transportation, heating, cooling and industry.
But desalination is an energy-intensive and expensive process, which concerns those wanting to expand its application. Other methods for purifying water include distillation and reverse osmosis. Distillation, or heating a mixture to extract volatile components that condense, requires a significant amount of energy.
Now, a team of experimentalists led by the Department of Energy’s Oak Ridge National Laboratory has demonstrated an energy-efficient desalination technology that uses a porous membrane made of strong, slim graphene, a carbon honeycomb one atom thick
The results are published in the current issue of Nature Nanotechnology.
“Our work is a proof of principle that demonstrates how you can desalinate saltwater using free-standing, porous graphene,” said Shannon Mark Mahurin of ORNL’s Chemical Sciences Division, who co-led the study with Ivan Vlassiouk in ORNL’s Energy and Transportation Science Division.
“It’s a huge advance,” said Vlassiouk, pointing out a wealth of water travels through the porous graphene membrane. “The flux through the current graphene membranes was at least an order of magnitude higher than [that through] state-of-the-art reverse osmosis polymeric membranes.”
Making pores in the graphene is key. Without these holes, water cannot travel from one side of the membrane to the other. The water molecules are simply too big to fit through graphene’s fine mesh. But, like a filter, if you poke holes in the mesh that are just the right size, the water molecules can get through.
Salt ions, in contrast, are larger than water molecules and cannot cross the membrane. The porous membrane allows osmosis, or passage of a fluid through a semipermeable membrane into a solution in which the solvent is more concentrated.
“If you have saltwater on one side of a porous membrane and freshwater on the other, an osmotic pressure tends to bring the water back to the saltwater side. But if you overcome that, and you reverse that, and you push the water from the saltwater side to the freshwater side—that’s the reverse osmosis process,” Mahurin explained.
Reverse osmosis, although a more energy-efficient process than the others still requires a lot of energy to work, and is the basis for the ORNL technology.
Today reverse-osmosis filters are typically polymers. A filter is thin and resides on a support. It takes significant pressure to push water from the saltwater side to the freshwater side.
“If you can make the membrane more porous and thinner, you can increase the flux through the membrane and reduce the pressure requirements, within limits,” Mahurin said. “That all serves to reduce the amount of energy that it takes to drive the process.”
With Graphene being only one-atom thick, yet its flexible and strong. Its mechanical and chemical stabilities make it promising in membranes for water separations.
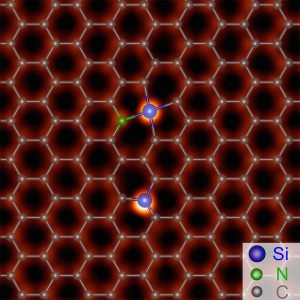
A porous graphene membrane could be more permeable than a polymer membrane; so separated water would drive faster through the membrane under the same conditions, the scientists reasoned. “If we can use this single layer of graphene, we could then increase the flux and reduce the membrane area to accomplish that same purification process,” Mahurin said.
To make graphene for the membrane, the researchers flowed methane through a tube furnace at 1,000 degrees Centigrade over a copper foil that catalyzed its decomposition into carbon and hydrogen.
The chemical vapor deposited carbon atoms that self-assembled into adjoining hexagons to form a sheet one atom thick.
[one_fourth]
[/one_fourth][three_fourth_last]
The researchers transferred the graphene membrane to a silicon nitride support with a micrometer-sized hole. Then the team exposed the graphene to oxygen plasma that knocked carbon atoms out of the graphene’s nanoscale “chicken wire lattice” to create pores.
Vlassiouk said making the porous graphene membranes used in the experiment is viable on an industrial scale, and other methods of production of the pores can be explored.
The longer the graphene membrane was exposed to the plasma, the bigger the pores that formed, and the more made.
The prepared membrane separated two water solutions salty water on one side, fresh on the other. The silicon nitride chip held the graphene membrane in place while water flowed through it from one chamber to the other.
The membrane allowed rapid transport of water through the membrane and rejected nearly 100 percent of the salt ions, e.g., positively charged sodium atoms and negatively charged chloride atoms.
They also found the optimal density of pores for desalination was one pore for every 100 square nanometers. “The more pores you get, the better, up to a point until you start to degrade any mechanical stability,” Mahurin said.
Research was sponsored by ORNL’s Laboratory Directed Research and Development Program. A portion of the work was conducted at the CNMS, a DOE Office of Science User Facility at ORNL.